"Green" hydrogen is a crime against the laws of physics
But the real crime is the massive misallocation of capital that will be spent chasing subsidies in a vain attempt to reach Net Zero.
There is no perpetual energy machine
The concept of taking the products of combustion and rearranging the molecules to recreate the original fuel like some perpetual energy creation machine sounds very appealing, but it comes up against one unbreakable law of nature.
The chemical energy required to reverse a reaction is the same as the energy released by that reaction. So, the splitting of water into hydrogen and oxygen consumes the same amount of chemical energy as that released when hydrogen burns with oxygen to make water.
That same rule is universal, whether making hydrogen or any other synthetic fuel.
However, none of the reactions can take place with 100% efficiency, so in real life, the energy consumed to make hydrogen from water is always more than the energy contained in the hydrogen that is produced.
In the case of electrolytic fuel cells operating on renewable power, the efficiency is around 60%.
When the hydrogen is converted back to electricity in a fuel cell or a turbine, the efficiency is about 50%. So, the whole conversion from electricity to hydrogen and back consumes about 3 kWh to produce 1 kWh of energy.
That fact alone should rule out electrolytic hydrogen as a potential fuel for light-duty transport such as personal cars, and certainly as a fuel for building heat where it must compete against heat pumps that can produce as much as 4 kWh of heat output for 1 kWh of input.
There is no such thing as free electricity from surplus renewables
Supporters of hydrogen claim that it can be produced cheaply by using “surplus” electricity that would otherwise be curtailed when wind and solar production is higher than demand.
However, the return on investment in a solar plant or wind farm depends on being able to sell the electricity it produces. Most of the renewable power produced today is sold at fixed prices or subsidized to make up the difference between market prices and the fixed contract price.
If the electricity is given away free of charge to the hydrogen plant, either the consumer or the taxpayer will have to carry the cost of the subsidy. Alternatively, if the producer is not paid for the curtailed energy, the rate charged for the remaining electricity will need to increase.
The electricity isn’t free at all, the cost is simply moved onto someone else.
Operating hydrolyzers intermittently is not viable
Another problem with electrolytic “green” hydrogen is the viability of operating hydrolyzers intermittently. This is not a technical problem though it does restrict the type of hydrolysers that can be used. PEM hydrolyzers are the technology of choice because they can operate at reasonably constant efficiency at anywhere from 0 to 100% load. However, they are not the cheapest or most efficient technology.
The problem is one of economics. A significant portion of the cost of hydrogen goes to finance and pay back the capital invested. The cost per unit of production rises steeply if the plant is limited in the number of hours and the input power level at which it can be operated.
But if the surplus renewable power is supplemented by grid power, then the hydrogen is not “green”. Making electrolytic hydrogen with electricity that has been generated using gas or coal produces 3 to 5 times more carbon emissions than making hydrogen by steam methane reforming. So, running a hydrolyzer on renewables when available and reverting to grid power at other times does not produce “green” hydrogen.
As wind and solar-powered electricity systems grow to the point where there are regular surpluses, those surpluses will be intermittent and highly varied in size. At times, there will be surpluses that exceed the capacity of the hydrolyzers, and power will still have to be curtailed. At other times, there will be surpluses that fall short of the capacity of the hydrolyzers, and they must operate at partial load or not at all.
Most of the studies and cost projections assume a utilization rate of about 50% as a reasonable target.
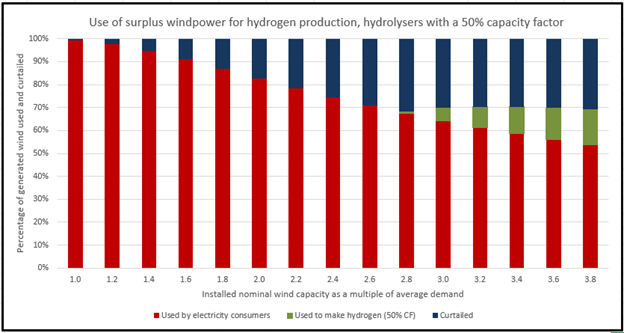
In a previous article, I analyzed 37 years of wind data from the UK and concluded that a hydrolyzer plant operating on surplus wind power could only achieve 50% utilization if wind power generation has grown to the point where 30% of the power is curtailed. The chart below is from that article:
The 30% curtailment rule continues to apply as more wind capacity and larger hydrolyzers are added.
In a solar-dominated system, the hydrolysers can never achieve 50% utilization. The whole concept of soaking up surplus renewables to produce hydrogen has serious limitations.
Hydrolyzers do not solve the problem of what to do with surplus power from renewables.
Costs of <$2/Kg are needed but are not achievable without massive subsidies
Supporters of electrolytic hydrogen hold out hopes of cheaper hydrolyzers that can be economically operated with lower capacity factors. The International Energy Agency, which seems to have transformed itself into a shill for the renewables industry has produced, seemingly out of thin air, a forecast cost of $400 to $700/Kw for future hydrolysers. Hydrolyzers in that price range can indeed be bought from China, but the cost of the hydrolyzer equipment is only a portion of the total cost of the project. The balance of the plant includes transformers and rectifiers for the power system, water treatment, dryers, compressors, coolers, storage and all the associated buildings, foundations, access roads etc., as well as financing costs.
This is an artist’s rendering of the proposed 55 MW Hunter Valley hydrogen project in Australia.
The cost was estimated at $2,600/MW before being cancelled due to poor economics and an uncertain market. The hydrolyzers would be located in that brown building in the top left of the picture. As you can see, they are a relatively small part of the total project. Trimming the cost of the hydrolyzer equipment by future mass production doesn’t reduce the overall project cost in the same proportion.
A recent study, sponsored by the Netherlands Ministry of Economic Affairs and Climate Policy, puts the cost of hydrolyser projects, based on an evaluation of 14 projects currently under development, at $3,050/Kw for a 100 Mw hydrolyser project and $2,630/Kw for a 200 MW project.
This chart, from the referenced Netherlands study, puts the levelized cost of hydrogen at €13.69 ($15.33) per Kg. It includes the cost of electricity and the tariff for connecting to the grid, as well as a smaller tariff for connecting to a hydrogen piping network.
That is where the costs of electrolytic hydrogen currently stand, and long way from where they need to be to make the projects viable. $15.33 per Kg equates to $393/MWh, more than ten times the cost of natural gas in Europe. Electricity produced from that hydrogen will cost ~$1,000/MWh once the cost of the storage systems and generating equipment have been included.
The UK has recently received bids at weighted average strike prices of £241/MWh ($320/MWh) for “green” electrolytic hydrogen, which equates to $12.48 per Kg. That price isn’t fixed, it will be adjusted for inflation going forward. The contract also includes a bonus a mechanism for increasing the strike price if the hydrogen is sold at more than 1.2 times the prevailing price of natural gas, and a mechanism for topping up the price if the quantity of hydrogen produced is less than half the target volume.
Those prices do not include the cost of storing large quantities of hydrogen or the cost of delivery.
I have seen forecasts of cheap hydrogen from renewables. Most of those forecasts come from vested interests and subsidy chasers, or from academics who don’t have any experience of cost estimating for real projects. Most are wishful thinking, targeting a “nice to have” price that meets a specific requirement, rather than a realistic price.
The prices I have quoted above are for real projects, without subsidies.
Hydrogen penetration will depend on costs
The chart below comes from a publication by the Hydrogen Council. It is an evaluation of the price points at which hydrogen becomes competitive for various applications.
As you can see from the chart, the real prices estimated for electrolytic hydrogen projects that are planned for construction in the next three years are unlikely to be competitive for anything except as a replacement for existing uses of hydrogen.
There is no compelling reason to assume that those prices can be significantly lower in future, certainly not low enough to compete for transportation or building heat, without massive subsidies.
Storage is a major restriction
Some hydrogen supporters claim that it will be cheap because it can be produced locally, eliminating distribution costs.
This brings up another major problem with “green” hydrogen. A kilogram of hydrogen at ambient pressure and temperature occupies 11.1 cu. m. (~400 cu.ft.). It must be compressed to a very high pressure for storage or transport. Storage tanks are usually 350 to 700 bar pressure and weigh 10 to 12 times the weight of the hydrogen that they contain.
This is a picture of one of the hydrogen storage tanks being transported to the Iberdrola electrolytic hydrogen facility in Spain. The tank is made of 40 mm thick high-strength steel, it is 2.5 meters in diameter and 20 meters tall. There are eleven tanks at the facility. The total energy stored in all 11 tanks together, is equal to the energy stored in one road tanker of diesel fuel. The cost of above-ground storage for hydrogen is astronomical.
“Green” hydrogen produced from wind and solar is intermittent and seasonal, both wind and solar tend to overproduce in spring when demand is low. Wind can be absent for long periods and can underproduce for periods of a year or more. But hydrogen demand does not follow the same pattern, most users operate 24 hrs/day, 350+ days/year.
Any attempt to make green hydrogen from wind and solar needs to have a backup supply or massive storage to match supply with demand. The Iberdrola facility, for example, is a small addition to a much larger hydrogen plant that uses steam methane reforming to produce most of its hydrogen. It can operate without the “greenwashing” hydrolyzer plant.
The only viable option for high-volume storage is the underground salt cavern, so large-scale hydrogen storage can only be located where the geology is suitable for underground storage.
Distribution by road over long distances is prohibitively expensive because a typical hydrogen fuel tanker can only carry about 600 Kg of hydrogen, it takes about 50 hydrogen trucks to replace one gasoline tanker truck. Hydrogen can be liquified for bulk distribution, but liquefaction consumes almost 1/3 of the contained energy, further increasing costs and reducing efficiency.
That’s why the majority of hydrogen plants (mostly using steam methane reforming) are situated close to the end users.
Any transition to hydrogen as a transport fuel will need a network of pipes to distribute the hydrogen to the end users. Pipes carrying hydrogen must be bigger than natural gas pipes to transport the equivalent energy. There is also an issue with hydrogen embrittlement. Hydrogen is a very small molecule, it can penetrate steel at welds and cause the steel over time, to become brittle and possibly fracture. It is therefore unlikely that existing gas pipelines can be repurposed for hydrogen.
The range of hydrogen cars is only marginally better than battery electric cars
The storage problem also arises at the individual transport level. The fuel tanks on hydrogen fuel cell vehicles are made from carbon fibre to minimize their weight. But on a car, the volume of the tank limits the amount of fuel that can be carried. The Toyota Mirai has two large fuel tanks which together, provide a range of only 402 miles. That range is only slightly higher than currently available BEV cars at similar price levels.
Given the high cost of “green” fuel, the limited range, and the lack of fuel stations, it is very unlikely that fuel cell vehicles will ever compete with battery electric vehicles for emission-free personal transport.
Advances in battery technology could also eliminate hydrogen as an option for emission-free commercial transport except for a small minority of very heavy-duty, long-distance applications.
Hydrogen has other drawbacks apart from economics.
Hydrogen is a dangerous gas. It will combust at anywhere between 4% and 75% concentration in air and will explode at between 18% and 59%. It is much lighter than air and disperses quickly, but indoors it can easily accumulate to combustible or explosive levels, and it takes very little energy to set it alight.
Hydrogen is a very small molecule, and it is usually stored and transported under high pressure. It can leak easily through joints and valves. Leaks are hard to detect because it is a colourless, odourless gas. Natural gas has traces of smelly gas added to make it detectable, but hydrogen is so light that it separates from any known odorant gas, so it can’t be made detectable by smell.
Though not itself a greenhouse gas, hydrogen in the atmosphere inhibits the decomposition of methane and other greenhouse gases and could be up to 100 times more potent than CO2 over a 10-year time frame. So, we have a molecule so small, that it can even leak into a steel weld, stored and transported under tremendous pressure. Does anyone believe that we will be able to control leaks?
The whole concept of “green” hydrogen made from surplus renewables is a crime against the laws of physics. But the real crime is the massive misallocation of capital that will be spent chasing subsidies for “green” hydrogen in a vain attempt to pursue an impossible and unnecessary Net Zero emissions target.






Does it make more sense to produce hydrogen using nuclear power?
Frankly, I can’t think of anything more hopeless than green hydrogen. Maybe flywheels for energy storage could be even worse. Where does such ignorance of basic physics come from? 🙄